FDA says Reynolds withdraws modified-risk applications for 6 Camel Snus styles
By A Mystery Man Writer
Last updated 23 May 2024

The Food and Drug Administration disclosed Thursday that R.J. Reynolds Tobacco Co. has requested the withdrawal of modified-risk tobacco product applications for six Camel Snus flavored products.
In Tunisia, more than 50 people have been in prison without trial for months for alleged conspiracy against state security or under a decree punishing the spreading of false information. Most of them are political opponents of President Kais Saied and the Ministry of justice has not been commenting on the cases publicly. For the families and lawyers of the prisoners, waiting for trials is becoming more and more trying. Lilia Blaise, Hamdi Tlili and Fadil Aliriza report.
In Tunisia, more than 50 people have been in prison without trial for months for alleged conspiracy against state security or under a decree punishing the spreading of false information. Most of them are political opponents of President Kais Saied and the Ministry of justice has not been commenting on the cases publicly. For the families and lawyers of the prisoners, waiting for trials is becoming more and more trying. Lilia Blaise, Hamdi Tlili and Fadil Aliriza report.

FDA spreads confusion about nicotine and smoking - The Counterfactual

PDF) Changes in Biomarkers of Exposure and Subjective Effects When Smokers Switch to Dual Use of Cigarettes and Either Snus or a Dissolvable Tobacco Product: A Summary of Three Clinical Studies

New Camel SNUS Ads: Let it Snus, FDA! - SnusCENTRAL

FDA's Scientific Advisory Committee Advances Modified Risk Classification for Camel Snus
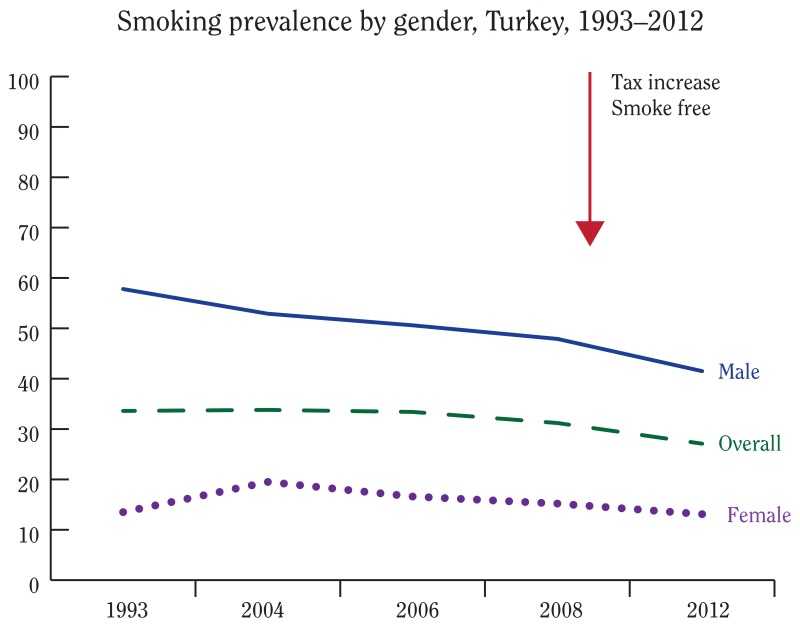
Current Status of Tobacco Control - The Health Consequences of Smoking—50 Years of Progress - NCBI Bookshelf

R.J. Reynolds Tobacco makes significant step on tobacco harm reduction; FDA to continue its evaluation on modified-risk claims for Camel Snus

Smoking, Tobacco and Cigarette News

FDA Grants First-Ever Modified Risk Orders to General Snus

MRTPs for Camel Snus – Tobacco Reporter

FDA's Scientific Advisory Committee Advances Modified Risk Classification for Camel Snus

R.J. Reynolds Pivots to New Cigarette Pitches as Flavor Ban Takes Effect - The New York Times
Recommended for you
-
 Customized Snus Can, Snus Container, Personalized Snus Box, Dip Can, Gift for Dip User, Gift for Snus User, Gift for Him, Metal Zyn Can23 May 2024
Customized Snus Can, Snus Container, Personalized Snus Box, Dip Can, Gift for Dip User, Gift for Snus User, Gift for Him, Metal Zyn Can23 May 2024 -
 Icetool Tri Can for portion snus - Silver - Divider Snus Can – Icetool snus accessories23 May 2024
Icetool Tri Can for portion snus - Silver - Divider Snus Can – Icetool snus accessories23 May 2024 -
 Icetool Tri Can for portion snus - Black with navy blue leather - Aluminum – Icetool snus accessories23 May 2024
Icetool Tri Can for portion snus - Black with navy blue leather - Aluminum – Icetool snus accessories23 May 2024 -
 China CNC Custom Parts Aluminium Slim Can Silver Catch Lid High Quality Snus Cans and Portioners Snus Accessories - China Snus Cans and Snussie Can price23 May 2024
China CNC Custom Parts Aluminium Slim Can Silver Catch Lid High Quality Snus Cans and Portioners Snus Accessories - China Snus Cans and Snussie Can price23 May 2024 -
 Buy Icetool Slim Can - Black at23 May 2024
Buy Icetool Slim Can - Black at23 May 2024 -
 Empty 5-pack Thor's Hammer Loose Snus Can23 May 2024
Empty 5-pack Thor's Hammer Loose Snus Can23 May 2024 -
 Closeup Of White Swedish Snus Can And Portion Snuff Pouches Viewed Through A Magnifying Glass Against A White Background Stock Photo - Download Image Now - iStock23 May 2024
Closeup Of White Swedish Snus Can And Portion Snuff Pouches Viewed Through A Magnifying Glass Against A White Background Stock Photo - Download Image Now - iStock23 May 2024 -
 IceTool Mini Can Silver - MyNicco23 May 2024
IceTool Mini Can Silver - MyNicco23 May 2024 -
 The Snussie Can - 24K23 May 2024
The Snussie Can - 24K23 May 2024 -
 How to Use Snus - Snus usage of loose and pouches23 May 2024
How to Use Snus - Snus usage of loose and pouches23 May 2024
You may also like
-
 We R Memory Keepers Button Press Backer Key-chain-Kit Makes 3 (15 pieces) 661074 by American Crafts23 May 2024
We R Memory Keepers Button Press Backer Key-chain-Kit Makes 3 (15 pieces) 661074 by American Crafts23 May 2024 -
 Book Embosser Personalized Book Stamp Custom Embosser Customized Your Design Stamp Gift for Library Books Business Cards Paper Notebooks23 May 2024
Book Embosser Personalized Book Stamp Custom Embosser Customized Your Design Stamp Gift for Library Books Business Cards Paper Notebooks23 May 2024 -
 Poly-Fil Premium Polyester Fiber Fill 10lb box by Poly-Fil | Joann x Ribblr23 May 2024
Poly-Fil Premium Polyester Fiber Fill 10lb box by Poly-Fil | Joann x Ribblr23 May 2024 -
 Foam Bricks Miniature23 May 2024
Foam Bricks Miniature23 May 2024 -
 Pen + Marker Holder (48) – Big Blue Laser Designs23 May 2024
Pen + Marker Holder (48) – Big Blue Laser Designs23 May 2024 -
 Black Book PNG Clipart - Best WEB Clipart23 May 2024
Black Book PNG Clipart - Best WEB Clipart23 May 2024 -
 Giant Light Bright Lite Brite Light up Peg Board 4x8 Jumbo Colorful Entertainment Activity23 May 2024
Giant Light Bright Lite Brite Light up Peg Board 4x8 Jumbo Colorful Entertainment Activity23 May 2024 -
 Amerikan Body Art Face Painting Glitter Poof - Sparkle White — Jest Paint - Face Paint Store23 May 2024
Amerikan Body Art Face Painting Glitter Poof - Sparkle White — Jest Paint - Face Paint Store23 May 2024 -
 Neenah Creative Collection Classics Specialty Cardstock Starter23 May 2024
Neenah Creative Collection Classics Specialty Cardstock Starter23 May 2024 -
 Adam Gleave23 May 2024
Adam Gleave23 May 2024